Our Team Members' Publications
In this section, we are happy to share with you the publications of our team members that you may find helpful. They may not necessarily be affiliated with Aropha.
10. Predicting Heavy Metal Adsorption on Soil with Machine Learning and Mapping Global Distribution of Soil Adsorption Capacities
Publication
Yang, H.; Huang, K.; Zhang, K.; Weng, Q.; Zhang, H.; Wang, F., Predicting Heavy Metal Adsorption on Soil with Machine Learning and Mapping Global Distribution of Soil Adsorption Capacities. Environ. Sci. Technol. 2021, 55, (20), 14316-14328. https://doi.org/10.1021/acs.est.1c02479
Abstract
Studying heavy metal adsorption on soil is important for understanding the fate of heavy metals and properly assessing the related environmental risks. Existing experimental methods and traditional models for quantifying adsorption, however, are time-consuming and ineffective. In this study, we developed machine learning models for the soil adsorption of six heavy metals (Cd(II), Cr(VI), Cu(II), Pb(II), Ni(II), and Zn(II)) using 4420 data points (1105 soils) extracted from 150 journal articles. After a comprehensive comparison, our results showed that the gradient boosting decision tree had the best performance for a combined model based on all the data. The Shapley additive explanation method was used to identify the feature importance and the effects of these features on the adsorption, based on which six independent models were developed for the six metals to achieve better model performance than the combined model. Using these independent models, the global distribution of heavy metal adsorption capacities on soils was predicted with known soil properties. Reversed models, including one combined model for all the six metals and six independent models, were also built using the same data sets to predict the heavy metal concentration in water when the adsorbed amount is known for a soil/sediment.
Source: https://doi.org/10.1021/acs.est.1c02479
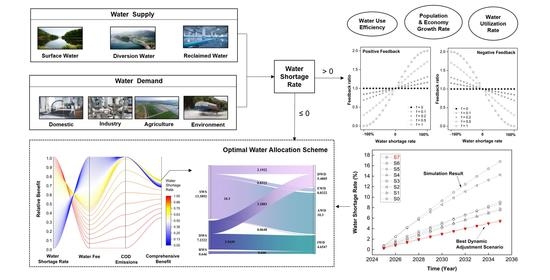
9. System Dynamics-Multiple Objective Optimization Model for Water Resource Management: A Case Study in Jiaxing City, China
Publication
Zhou, X.; Wang, F.; Huang, K.; Zhang, H.; Yu, J.; Han, A. Y., System dynamics-multiple objective optimization model for water resource management: A case study in Jiaxing City, China. Water 2021, 13, (5), 671. https://doi.org/10.3390/w13050671
Abstract
Predicting and allocating water resources have become important tasks in water resource management. System dynamics and optimal planning models are widely applied to solve individual problems, but are seldom combined in studies. In this work, we developed a framework involving a system dynamics-multiple objective optimization (SD-MOO) model, which integrated the functions of simulation, policy control, and water allocation, and applied it to a case study of water management in Jiaxing, China to demonstrate the modeling. The predicted results of the case study showed that water shortage would not occur at a high-inflow level during 2018–2035 but would appear at mid- and low-inflow levels in 2025 and 2022, respectively. After we made dynamic adjustments to water use efficiency, economic growth, population growth, and water resource utilization, the predicted water shortage rates decreased by approximately 69–70% at the mid- and low-inflow levels in 2025 and 2035 compared to the scenarios without any adjustment strategies. Water allocation schemes obtained from the “prediction + dynamic regulation + optimization” framework were competitive in terms of social, economic and environmental benefits and flexibly satisfied the water demands. The case study demonstrated that the SD-MOO model framework could be an effective tool in achieving sustainable water resource management.
Source: https://doi.org/10.3390/w13050671
8. An improved weighted index for the assessment of heavy metal pollution in soils in Zhejiang, China
Publication
Yang, H.; Wang, F.; Yu, J.; Huang, K.; Zhang, H.; Fu, Z., An improved weighted index for the assessment of heavy metal pollution in soils in Zhejiang, China. Environ. Res. 2020, 110246. https://doi.org/10.1016/j.envres.2020.110246
Abstract
Soil heavy metal pollution assessment is an important procedure in soil quality and ecological risk management, for which different mathematical models have been developed. However, these models have often failed to consider the characteristics of both heavy metals and the polluted sites. In this study, we analyzed the concentrations of seven heavy metals in soils in Zhejiang Province, China, and developed an improved weighted index (IWI) model to evaluate pollution levels. In contrast to traditional models, weights were assigned to different heavy metals using statistical tools, including hierarchical cluster analysis and principal component analysis. Of the 89 sites, 61.8% were considered unpolluted with IWI values < 1; 32.58% were slighted polluted with IWI values from 1 to 2, and only 2.25% of the sites were seriously polluted with IWI values > 3. The IWI results agree well with two traditional integrated index models, but can be also applied to much wider heavy metal concentration ranges. Possible pollution sources were then proposed based on the IWI model. The IWI overcame several shortcomings of the traditional indices and could be very beneficial for assessing heavy metal pollution in soil. Overall, this study developed a new model for soil pollution assessment and soil ecological risk management and comprehensively evaluated the current pollution status of soil surrounding potable surface water sources in Zhejiang Province, China.
Source: https://doi.org/10.1016/j.envres.2020.110246
7. Galvanic oxidation processes (GOPs): An effective direct electron transfer approach for organic contaminant oxidation
Publication
Huang, K. Z.; Zhang, H., Galvanic oxidation processes (GOPs): An effective direct electron transfer approach for organic contaminant oxidation. Sci. Total Environ. 2020, 743, 140828. https://doi.org/10.1016/j.scitotenv.2020.140828
Abstract
The activation of peroxymonosulfate (PMS) for organic contaminant oxidation usually relies on the formation of reactive oxygen species (ROSs). However, the ubiquitous anions and natural organic matter can easily scavenge ROSs and/or PMS, resulting in lower efficiencies and/or the formation of toxic byproducts. Relying on the unique long-distance electron transfer property, the recently developed Galvanic Oxidation Process (GOP) successfully achieved bisphenol A (BPA) degradation when BPA and PMS were physically separated in two reactors. In this study, we systematically investigated the performance of GOP at different PMS or BPA concentrations, pH, and ionic strength (IS) in both PMS and BPA solutions. The kinetic modeling employing the Langmuir-Hinshelwood model at different BPA concentrations suggested that although BPA and PMS were physically separated, the oxidation of the adsorbed BPA and reduction of the adsorbed PMS still followed a similar mechanism to that in traditional heterogeneous catalytic processes. The anions in the target water showed little impact on BPA degradation; higher IS enhanced the solution conductivity but inhibited BPA and electrode interactions, resulting in increased and then decrease BPA degradation rate. The electrodes presented high stability with a rate increase of 12% after 13 times of uses, and their hydration significantly facilitated BPA degradation but reduced the current by decreasing the potential difference between the anode and cathode. The graphite sheet itself without catalyst coating was also capable of shuttling electrons, while the use of a graphite fiber anode increased the BPA degradation by near 100% because of the larger surface area. The developed continuous stirred-tank reactor coupled with GOP (CSTR-GOP) achieved stable BPA degradation in less than 35 min and its scaling up is promising for future applications.
Source: https://doi.org/10.1016/j.scitotenv.2020.140828
6. Highly efficient bromide removal from shale gas produced water by unactivated peroxymonosulfate for controlling disinfection byproduct formation in impacted water supplies
Publication
Huang, K. Z.; Zhang, H., Highly efficient bromide removal from shale gas produced water by unactivated peroxymonosulfate for controlling disinfection byproduct formation in impacted water supplies. Environ. Sci. Technol. 2020, 54, (8), 5186-5196. https://doi.org/10.1021/acs.est.9b06825
Abstract
Shale gas extraction processes generate a large amount of hypersaline wastewater, whose spills or discharges may significantly increase the bromide levels in downstream water supplies and result in the formation of brominated disinfection byproducts (DBPs) upon chlorination. Although a few studies have investigated selective bromide removal from produced water, the low removal efficiencies and complex system setups are not desirable. In this study, we examined a simple cost-effective approach for selective bromide removal from produced water relying on the oxidation by unactivated peroxymonosulfate. More than 95% of bromide was removed as Br2(g) in less than 10 min under weakly acidic conditions without significant formation of Cl2(g) even when the chloride concentration was more than 2 orders of magnitude higher. A kinetic model considering the involved reactions was then developed to describe the process well under various reaction conditions. The organic compounds in the produced water neither noticeably lowered the bromide removal efficiency nor reacted with the halogen species to form halogenated byproducts. The tests in batch and continuously stirred tank reactor systems suggested that it was feasible to achieve both high bromide removal and neutral effluent pH such that further pH adjustment was not necessary before discharge. After the treatment, the effect of the produced water on DBP formation was largely eliminated.
Source: https://doi.org/10.1021/acs.est.9b06825
5. Direct electron-transfer-based peroxymonosulfate activation by iron-doped manganese oxide (δ-MnO2) and the development of Galvanic Oxidation Processes (GOPs)
Publication
Huang, K. Z.; Zhang, H., Direct electron-transfer-based peroxymonosulfate activation by iron-doped manganese oxide (δ-MnO2) and the development of Galvanic Oxidation Processes (GOPs). Environ. Sci. Technol. 2019, 53, (21), 12610-12620. https://doi.org/10.1021/acs.est.9b03648
Abstract
Manganese oxides have been recently investigated as excellent catalysts for peroxymonosulfate (PMS) activation, and the reported mechanisms are mostly forming reactive oxygen species (ROSs). This study investigated the use of iron-doped manganese oxide, synthesized via air oxidation under strong alkaline conditions. The oxidation of three substrates was affected by their adsorption at the catalyst surface, solution pH, and co-solutes. Common ROS scavengers inhibited the oxidation of bisphenol A (BPA), suggesting the possible involvement of ROSs; however, the PMS decomposition tests with and without BPA and the comparison with a 1O2-generation system ruled out the formation of ROSs and pointed to direct electron transfer between the adsorbed BPA and complexed PMS as the mechanism. To prove this mechanism, the catalyst was coated to graphite sheets and a galvanic oxidation process (GOP) was developed to separate BPA and PMS into two half cells. Upon PMS addition into one cell, BPA was quickly oxidized in the other cell, confirming the occurrence of electron transfer. The GOP system successfully degraded BPA in both surface water and hypersaline shale gas-produced water. Overall, this study developed a new catalyst for PMS activation and unveiled the advantages and potential applications of electron shuttling catalysts.
Source: https://doi.org/10.1021/acs.est.9b03648
4. Formation of disinfection by-products under influence of shale gas produced water
Publication
Huang, K. Z.; Xie, Y. F.; Tang, H. L., Formation of disinfection by-products under influence of shale gas produced water. Sci. Total Environ. 2019, 647, 744-751. https://doi.org/10.1016/j.scitotenv.2018.08.055
Abstract
Accidental spills and surface discharges of shale gas produced water could contaminate water resources and generate health concerns. The study explored the formation and speciation of disinfection by-products (DBPs) during chlorination of natural waters under the influence of shale gas produced water. Results showed the presence of produced water as low as 0.005% changed the DBP profile measurably. A shift to a more bromine substitution direction for the formation of trihalomethanes, dihaloacetic acids, trihaloacetic acids, and dihaloacetonitriles was illustrated by exploring the individual DBP species levels, bromine substitution factors, and DBP species fractions, and the effect was attributable to the introduction of bromide from produced water. The ratio of dichloroacetic and trichloroacetic acids also increased, which was likely affected by different bromination degrees at elevated bromide concentrations. Increasing blend ratios of produced water enhanced the formation of DBPs, especially the brominated species, while such negative effects could be alleviated by pre-treating the produced water with ozone/air stripping to remove bromide. The study advances understandings about the impacts of produced water spills or surface discharges regarding potential violation of Stage 2 DBP rules at drinking water treatment facilities.
Source: https://doi.org/10.1016/j.scitotenv.2018.08.055
3. Temperature and desorption mode matter in capacitive deionization process for water desalination
Publication
Huang, K. Z.; Tang, H. L., Temperature and desorption mode matter in capacitive deionization process for water desalination. Environ. Technol. 2019, 1-8. https://doi.org/10.1080/09593330.2019.1611941
Abstract
Literature reporting temperature and desorption mode as factors of capacitive deionization (CDI) process for water desalination is rare. This study explored the impacts of four water temperatures (15°C, 25°C, 35°C, and 45°C), three salt concentrations (350, 1260, and 3100 mg/L), and three desorption modes (potential removal, short circuit, and polarity reversal) on performance of a ‘closed-loop’ CDI system. Results showed that a higher temperature promoted adsorption and desorption rates but impaired adsorption capacity. Polarity reversal could greatly expedite the desorption process compared to short circuit and potential removal. A promotional impact of concentration on CDI desalination could be explained by the formation of electrical double layers. The research also noted the earlier occurrence of re-adsorption at higher temperatures during polarity-reversal desorption. Strategies of increasing water temperature on short adsorption cycles and using an adjustable combination mode of polarity reversal and short circuit for desorption are implied for improving desalination efficiency and water recovery of CDI systems.
Source: https://doi.org/10.1080/09593330.2019.1611941
2. Impacts of shale gas production wastewater on disinfection byproduct formation: An investigation from a non-bromide perspective
Publication
Huang, K. Z.; Tang, H. L.; Xie, Y. F., Impacts of shale gas production wastewater on disinfection byproduct formation: An investigation from a non-bromide perspective. Water Res. 2018, 144, 656-664. https://doi.org/10.1016/j.watres.2018.07.048
Abstract
The rapid rise of shale gas development has triggered environmental and human health concerns due to its impacts on water resources, especially on disinfection byproduct (DBP) formation upon chlorination. Despite the recently reported results on bromide, the effects of non-bromide ions in production wastewater at extremely high levels are vaguely defined. In this study, we investigated the effects of production wastewater, with bromide and non-bromide species, on the formation of DBPs when production wastewater was spiked into surface waters at various percentages. Results showed that the introduction of debrominated production wastewater led to increased formation of some chlorinated DBP species in selected surface water and wastewater. As the spiking percentage of debrominated production wastewater increased, the chlorinated DBP species increased. The contributions of individual cations to DBP formation followed a sequence of magnesium > calcium > barium at 0.10% spiking percentage due to the different catalytic effects of their chelates with organic precursors. The study of anions suggested that the discharge of treated production wastewater containing elevated sulfate may further enhance DBP formation. The significance of this study lies in the fact that in addition to bromide concerns from production wastewater, non-bromide species also contributed to DBP formation. The gas production wastewater management decision should consider the negative impacts from both bromide and non-bromide species to better protect the receiving water resources.
Source: https://doi.org/10.1016/j.watres.2018.07.048
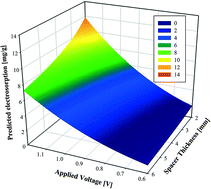
1. Relation between operating parameters and desalination performance of capacitive deionization with activated carbon electrodes
Publication
Liu, D.; Huang, K.; Xie, L.; Tang, H. L., Relation between operating parameters and desalination performance of capacitive deionization with activated carbon electrodes. Environ. Sci-Wat. Res. 2015, 1, (4), 516-522. https://doi.org/10.1039/C5EW00102A
Abstract
Electrosorptive batch experiments were conducted for the development of a statistical model to describe the relation between operating parameters and desalination performance of capacitive deionization (CDI) with activated carbon electrodes. Results showed that the statistical model reproduced the data well, with both regression and verification R2 values above 0.85. The exponents in the statistical model, shown as 2.79, −0.45, and 1.05 for the applied voltage, spacer thickness, and retention time, respectively, revealed the importance of each factor in the correspondingly examined range. In addition, the effects of interactions between the factors were judged as significant, which could be explained by the electrical double-layer theory. The influence of initial salt concentration could be best characterized by the Langmuir isotherm. This work demonstrates that direct modeling of desalination performance with operating parameters is feasible and the results can be used to guide practical engineering applications.