What Are the Aerobic Biodegradation Mechanisms?
Aerobic biodegradation mechanisms
Aerobic biodegradation is a natural process where complex organic substances are broken down into smaller and simpler compounds by the enzymes produced by the microorganisms when oxygen is present. It usually involves the metabolic and enzymatic processes with the final products of carbon dioxide and water.
The organic compounds can be either electron donors or acceptors depending on their oxidation states, while oxygen usually acts as an electron acceptor or being involved in the activation of the substrate via oxygenation reactions.1
The fundamental units of biodegradation are functional groups, as these are the ‘elements’ that undergo the transformation during the course of microbial catabolism.2 Each functional group may undergo several types of enzymatic transformations. Therefore, different compounds may show substantially different biodegradation behaviors. However, with about 40 most relevant functional groups found in the environmentally important compounds, and perhaps three to four transformation reactions for each, the University of Minnesota Biocatalysis/Biodegradation Database has been successfully collecting such information for two decades.3
On the other hand, the functional groups/substructures that are beneficial for biodegradation generally follow the order: ester, amide, anhydride > hydroxyl > carboxyl, epoxide, site of unsaturation > benzene ring, methyl, methylene, while the negative influences were molecular mass, branching, halogenation, and nitrogen heterocycles.4
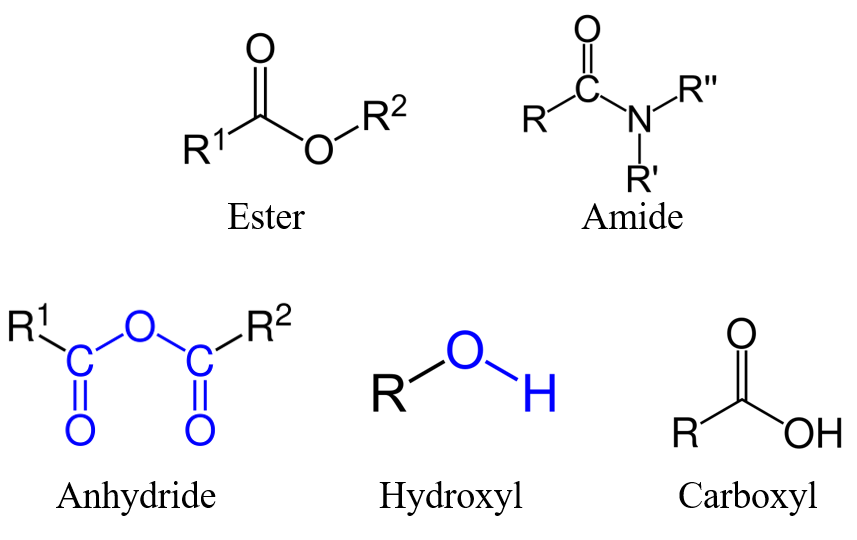
Figure 4. Example functional groups that are beneficial for biodegradation
For example, the initial attack on the aromatic ring is mostly assumed to be electrophilic in nature. Nitrogen ([-CN, -NO2, -NH3+, -CONH2]) and halogenic substituents strongly deplete the electron density of the aromatic ring and decrease the rate of biodegradation, while in the presence of hydroxyl and carboxyl groups was found to increase the biodegradation.5, 6
In addition, compounds that are already partially oxidized are generally considered to be more easily attacked than those which are not, and the initial degradation step is considered to be rate limiting step.6
References
- Cao, B.; Nagarajan, K.; Loh, K. C. Biodegradation of aromatic compounds: current status and opportunities for biomolecular approaches. Appl. Microbiol. Biotechnol. 2009, 85 (2), 207-28.
- Wackett, L. P.; Ellis, L. B. Predicting biodegradation. Environ. Microbiol. 1999, 1 (2), 119-124.
- Ellis, L. B.; Hershberger, C. D.; Wackett, L. P. The University of Minnesota Biocatalysis/Biodegradation Database: specialized metabolism for functional genomics. Nucleic Acids Res. 1999, 27 (1), 373-376.
- Boethling, R. S.; Gregg, B.; Frederick, R.; Gabel, N. W.; Campbell, S. E.; Sabljic, A. Expert systems survey on biodegradation of xenobiotic chemicals. Ecotoxicol. Environ. Saf. 1989, 18 (3), 252-267.
- Acharya, K.; Werner, D.; Dolfing, J.; Barycki, M.; Meynet, P.; Mrozik, W.; Komolafe, O.; Puzyn, T.; Davenport, R. J. A quantitative structure-biodegradation relationship (QSBR) approach to predict biodegradation rates of aromatic chemicals. Water Res. 2019, 157, 181-190.
- Acharya, K.; Werner, D.; Dolfing, J.; Meynet, P.; Tabraiz, S.; Baluja, M. Q.; Petropoulos, E.; Mrozik, W.; Davenport, R. J. The experimental determination of reliable biodegradation rates for mono-aromatics towards evaluating QSBR models. Water Res. 2019, 160, 278-287.