Biodegradation Test Method Overview
Biodegradability test (screening test) has been a common practice to investigate the environmental fate of newly developed materials once they are released into the environment.
Traditional experimental tests are conducted to investigate the biodegradability.
However, recent advances in computer science has provided numerous in-silico ways to predict the biodegradability based on machine learning models.
In this section, we will dive into both segments to take a quick look at how they can help us achieve biodegradability estimation (for both ready and inherent biodegradation).
Experimental test
For the experimental test (for ready and inherent biodegradation), the detailed system setups and procedures have been very well documented into different series of standard methods by different organizations, such as OECD, EPA, EU, and ISO.
A glance of different biodegradation test guidelines
Below is a glance of these documented standard methods (sorted in alphabetical order).
| Number | Guideline | Note | Principle | Endpoint |
|---|---|---|---|---|
| 1 | EPA OPPTS 835.3170 | Shake Flask Die-away Test | - | - |
| 2 | EPA OTS 796.3100 | - | - | - |
| 3 | OECD 306 (II) | Closed Bottle Test in Seawater | Closed Bottle Test in Seawater | - |
| 4 | ASTM D6731 | Lubricants or Lubricant Components in a Closed Respirometer | Closed Respirometer | - |
| 5 | ISO 14851 | Plastics Oxygen Consumption Closed Respirometer | Closed Respirometer | - |
| 6 | ASTM D5864 | Biodegradation of Lubricants or Their Components CO2 Evolution Test | CO2 Evolution | - |
| 7 | ASTM D6139 | Biodegradation of Lubricants or Their Components Using the Gledhill Shake Flask | CO2 Evolution | - |
| 8 | EPA OPPTS 835.3100 | CO2 Evolution | CO2 Evolution | - |
| 9 | ISO 14852 | Plastics CO2 Evolution | CO2 Evolution | - |
| 10 | OECD 306 (I) | Shake Flask Test in Seawater | DOC Die Away in Seawater | - |
| 11 | EPA OPPTS 835.3215 | Concawe Test | - | Inherent |
| 12 | EPA OPPTS 835.5045 | Modified SCAS Test for Insoluble and Volatile Chemicals | - | Inherent |
| 13 | OECD 302 C | Modified MITI Test (II) | Closed Respirometer | Inherent |
| 14 | OECD 302 D (proposed) | Closed Respirometer | Closed Respirometer | Inherent |
| 15 | EPA OPPTS 835.3200 | Zahn-Wellens / EMPA Test | DOC Die Away | Inherent |
| 16 | EPA OPPTS 835.3210 | Modified SCAS Test | DOC Die Away | Inherent |
| 17 | EPA OTS 795.45 | Modified SCAS Test | DOC Die Away | Inherent |
| 18 | EPA OTS 796.3340 | Modified SCAS Test | DOC Die Away | Inherent |
| 19 | EPA OTS 796.3360 | Modified Zahn-Wellens Test | DOC Die Away | Inherent |
| 20 | EU Method C.9 | Zahn-Wellens Test | DOC Die Away | Inherent |
| 21 | EU Method C.12 | Modified SCAS Test | DOC Die Away | Inherent |
| 22 | ISO 9887 | SCAS Test | DOC Die Away | Inherent |
| 23 | ISO 9888 | Zahn-Wellens Test | DOC Die Away | Inherent |
| 24 | OECD 302 A | Modified SCAS Test | DOC Die Away | Inherent |
| 25 | OECD 302 B | Zahn-Wellens / EMPA Test | DOC Die Away | Inherent |
| 26 | EPA OTS 796.3180 | Modified AFNOR Test | - | Ready |
| 27 | EPA OTS 796.3240 | Modified OECD Screening Test | - | Ready |
| 28 | EPA OTS 796.3200 | Closed Bottle Test | Closed Bottle Test | Ready |
| 29 | EPA OTS 796.3220 | Modified MITI Test (I) | Closed Bottle Test | Ready |
| 30 | EU Method C.4-E | Closed Bottle Test | Closed Bottle Test | Ready |
| 31 | EU Method C.4-F | MITI Test | Closed Bottle Test | Ready |
| 32 | EU Method C.5 | Biochemical Oxygen Demand | Closed Bottle Test | Ready |
| 33 | ISO 10707 | Closed Bottle Test | Closed Bottle Test | Ready |
| 34 | OECD 301 D | Closed Bottle Test | Closed Bottle Test | Ready |
| 35 | EPA OPPTS 835.3140 | CO2 Headspace Test | Closed Respirometer | Ready |
| 36 | EU Method C.4-D | Manometric Respirometry Test | Closed Respirometer | Ready |
| 37 | EU Method C.29 | CO2 Headspace Test | Closed Respirometer | Ready |
| 38 | ISO 9408 | Manometric Respirometry Test | Closed Respirometer | Ready |
| 39 | ISO 10708 | Two-Phase Closed Bottle Test | Closed Respirometer | Ready |
| 40 | ISO 14593 | CO2 Headspace Test | Closed Respirometer | Ready |
| 41 | OECD 301 C | Modified MITI Test (I) | Closed Respirometer | Ready |
| 42 | OECD 301 F | Manometric Respirometry Test | Closed Respirometer | Ready |
| 43 | OECD 310 | CO2 Headspace Test | Closed Respirometer | Ready |
| 44 | EPA OTS 796.3260 | Modified Sturm Test | CO2 Evolution | Ready |
| 45 | EU Method C.4-C | CO2 Evolution | CO2 Evolution | Ready |
| 46 | ISO 9439 | CO2 Evolution | CO2 Evolution | Ready |
| 47 | OECD 301 B | CO2 Evolution | CO2 Evolution | Ready |
| 48 | EU Method C.6 | Chemical Oxygen Demand | COD | Ready |
| 49 | EU Method C.4-A | DOC Die Away | DOC Die Away | Ready |
| 50 | EU Method C.4-B | Modified OECD Screening Test | DOC Die Away | Ready |
| 51 | ISO 7827 | DOC Die Away | DOC Die Away | Ready |
| 52 | OECD 301 A | DOC Die Away | DOC Die Away | Ready |
| 53 | OECD 301 E | Modified OECD Screening Test | DOC Die Away | Ready |
| 54 | OECD 303B | Simulation Test - Biofilms | Simulation | Simulation |
| 55 | ISO 11733 | Simulation Test - Activated Sludge Unit | Simulation (DOC/COD removal) | Simulation |
| 56 | OECD 303A | Simulation Test - Activated Sludge Unit | Simulation (DOC/COD removal) | Simulation |
Summary of different guidelines
The table above indicates that even though there are quite a large number of standard methods, they are mostly similar to each other in terms of principles and endpoints.
Among different principles, the DOC Die Away was found to be the most popular with 16 individual guidelines, followed by Closed Respirometer with 13 guidelines.
The least popular guidelines were DOC Die Away in Seawater, Closed Bottle Test in Seawater and COD, each having only one guideline.
| Number | Principle | Number of guidelines |
|---|---|---|
| 1 | DOC Die Away in Seawater | 1 |
| 2 | Closed Bottle Test in Seawater | 1 |
| 3 | COD | 1 |
| 4 | Simulation | 3 |
| 5 | Closed Bottle Test | 7 |
| 6 | CO2 Evolution | 8 |
| 7 | Closed Respirometer | 13 |
| 8 | DOC Die Away | 16 |
As for the endpoints of the tests, most guidelines are for Ready biodegradability test with a total number of 28, while 15 are designed for Inherent biodegradability tests, and only 3 for the Simulation tests..
| Number | Endpoint | Number of guidelines |
|---|---|---|
| 1 | Simulation | 3 |
| 2 | Inherent | 15 |
| 3 | Ready | 28 |
OECD methods
Among the different series of methods shown above, OECD methods gained the highest popularity in many countries.
In addition to above 301 and 302 series of tests for ready and inherent biodegradability tests, respectively, OECD also has a 303 series of test.
OECD methods were well developed to accommodate most of the biodegradation test requirements.
Generally, biodegradation tests are grouped into three tiers in OECD, including 301A-F as the first tier, 302A B C as the second tier, and 303A-B as the third tier.
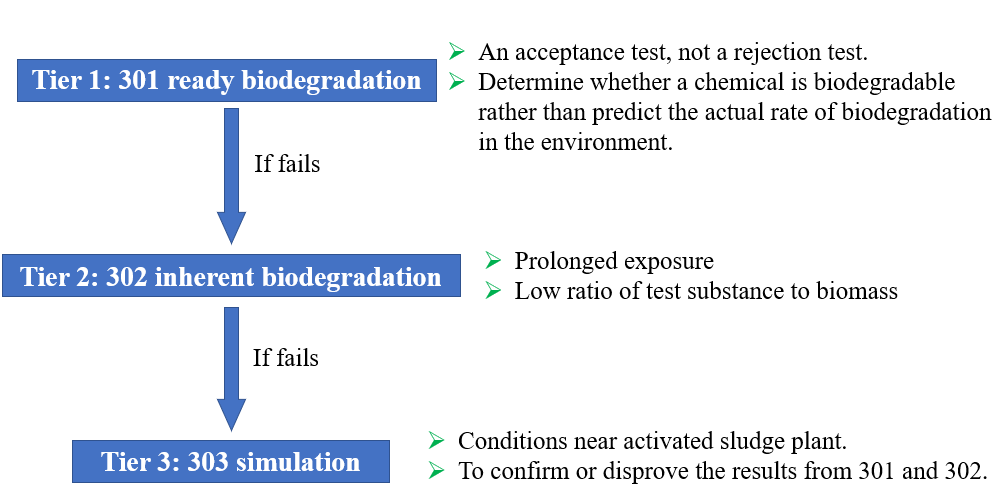
301 and 302 are designed for ready and inherent biodegradability test, respectively, under relatively stringent conditions (high substance to inoculum ratio), while 303 are for simulated biodegradation under identical conditions compared to the real activated sludge treatment processes.
The 301 and 302 methods are considered acceptance tests instead of rejection tests, meaning that if a sample fails these tests, it does not necessarily mean this compound cannot be degraded once it enters the environment. It could still be potentially biodegradable under more favorable conditions (e.g., higher microorganism concentration, and/or longer exposure time).
301 ready biodegradation tests: An acceptance test, not a rejection test. It selects chemicals that do not have to be tested further because high biodegradability is expected in sewage treatment plants. Measurement is based on nonspecific parameters like DOC, BOD, or CO2. Developed to devise screening methods to determine whether a chemical is potentially easily biodegradable, rather than to predict the actual rate, of biodegradation in the environment. A readily biodegradable material is assumed to be able to undergo rapid and ultimate biodegradation in the environment. Therefore no further investigation on the biodegradability, toxicity, or other environmental effects is normally required.
302 inherent biodegradability tests: Allow prolonged exposure of the test substance to microorganisms and a low ratio of test substance to biomass, which offers a better chance to obtain a positive result compared to tests for ready biodegradability. Biodegradation percentages above 20% may be regarded as evidence of inherent, primary biodegradability, whereas biodegradation percentages above 70% may be regarded as evidence of inherent, ultimate biodegradability.
303 simulation tests: Based on a simulation of the conditions existing in an activated sludge plant. This test should be used for any chemicals that did not pass the previous tests, either to confirm or disprove the first results obtained.
A test usually starts from the lowest tier of methods (301). If it fails, one can move on to higher tiers until a confident conclusion can be drawn.
Environmental risk assessment can be performed if a compound fails all these tests.
More information can be found in the OECD official document.
306 biodegradability in seawater: OECD 306 is an aerobic biodegradation test that determines the biodegradability of an organic material in seawater relying on the microorganisms originally present in seawater without the addition of a specific inoculum. Two methods are specified, i.e., (I)measuring dissolved organic carbon removal in a Shake Flask Method, and (II) determining dissolved oxygen consumption in a Closed Bottle Method. The Shake Flask Method allows a maximum of 60 days of test duration, while the Closed Bottle Method suggests 28 days because no further information can be normally gathered in an extended test.
ISO methods
In addition to OECD methods, there are also a number of ISO standard methods for aqueous aerobic biodegradability tests. We currently provide analysis of eight of them, i.e., ISO 9408, ISO 9439, ISO 9888, ISO 10707, ISO 11733, ISO 14851, ISO 14852, and ISO 16221. Some of them are equivalent to some of the OECD methods. Please note that ISO 14851 and 14852 are designed specifically for plastics.
ASTM D5864 and ASTM 6731
ASTM D5864 and D6731 are two ASTM methods specifically designed for the biodegradation test of lubricants or their components. ASTM D5864 measures the CO2 evolution, which is equivalent to the OECD 301B method. ASTM D6731 measures the oxygen consumption, which is equivalent to the OECD 301F.
ASTM6731 is our preferred method for lubricant test due to its simple system setup and high applicability.
Prediction using machine learning
Traditional standard methods for biodegradation tests are generally very time-consuming and labor-intensive. To overcome these drawbacks, a number of alternative approaches have been proposed in the literature to decrease the time and labor input (e.g., for BOD tests).1
With the increase of the documented experimental data as well as the development of data science in recent years, machine learning has been encouraged to develop predictive models for easy prioritization of newly developed compounds.2-5
At Aropha, a significant portion of our efforts have been dedicated to develop such predictive models. Please visit the "Machine Learning Prediction" page for more details.
Test methods we provide at Aropha
What we provide
At Aropha, we are dedicated to provide accurate but also cost-effective methods to meet our customers' needs at the lowest prices.
To achieve these goals, we are putting a significant amount of efforts to incorporate automation and other novel technologies for ready, inherent, and simulated biodegradability, and in-silico prediction using machine learning.
System setups based on closed bottle, closed respirometer, or CO2 evolution have the top levels of simplicity and applicability. Such methods are most commonly used. For a full list of the methods we provide, please check this page.
In addition to experimental tests, we also provide predictive models developed based on machine learning for cheap, quick, and reliable predictions of target compounds (with specific chemical structures). Please visit the page "Machine Learning Prediction" for more details.
References
- Jouanneau, S.; Recoules, L.; Durand, M. J.; Boukabache, A.; Picot, V.; Primault, Y.; Lakel, A.; Sengelin, M.; Barillon, B.; Thouand, G. Methods for assessing biochemical oxygen demand (BOD): a review. Water Res. 2014, 49, 62-82.
- Cheng, F.; Ikenaga, Y.; Zhou, Y.; Yu, Y.; Li, W.; Shen, J.; Du, Z.; Chen, L.; Xu, C.; Liu, G.; Lee, P. W.; Tang, Y. In silico assessment of chemical biodegradability. J. Chem. Inf. Model. 2012, 52 (3), 655-69.
- Mansouri, K.; Ringsted, T.; Ballabio, D.; Todeschini, R.; Consonni, V. Quantitative structure-activity relationship models for ready biodegradability of chemicals. J. Chem. Inf. Model. 2013, 53 (4), 867-78.
- Pizzo, F.; Lombardo, A.; Brandt, M.; Manganaro, A.; Benfenati, E. A new integrated in silico strategy for the assessment and prioritization of persistence of chemicals under REACH. Environ. Int. 2016, 88, 250-260.
- Fernandez, A.; Rallo, R.; Giralt, F. Prioritization of in silico models and molecular descriptors for the assessment of ready biodegradability. Environ. Res. 2015, 142, 161-8.